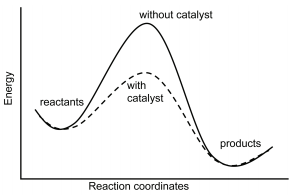
29.8: A Catalyst Affects the Mechanism and Activation Energy - Chemistry LibreTexts
Homogeneous catalysis refers to reactions in which the catalyst is in solution with at least one of the reactants whereas heterogeneous catalysis refers to reactions in which the catalyst is present …
Homogeneous catalysis refers to reactions in which the catalyst is in solution with at least one of the reactants whereas heterogeneous catalysis refers to reactions in which the catalyst is present in a different phase, usually as a solid, than the reactants.
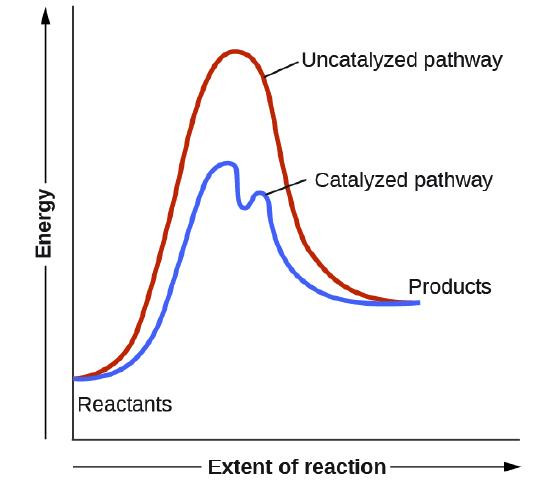
4.6: Catalysis - Chemistry LibreTexts

What can a catalyst do and what it cannot do?

6.10: Energies, Kinetics, and Catalysts - Chemistry LibreTexts

Chemical Kinetics: SR - 12 Class Chemistry Vol-2, PDF, Reaction Rate
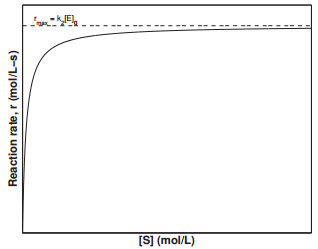
29.9: The Michaelis-Menten Mechanism for Enzyme Catalysis - Chemistry LibreTexts
12.7: Catalysis - Chemistry LibreTexts
12.7: Catalysis - Chemistry LibreTexts
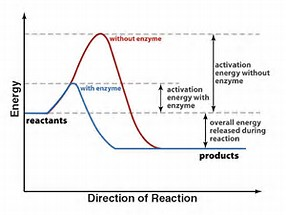
Does a catalyst change the thermodynamics of a chemical reaction?

What Is a Catalyst? Understand Catalysis

Sequential Transformation of Terminal Alkynes to 1,3-Dienes by a Cooperative Cobalt Pyridonate Catalyst
12.7: Catalysis - Chemistry LibreTexts
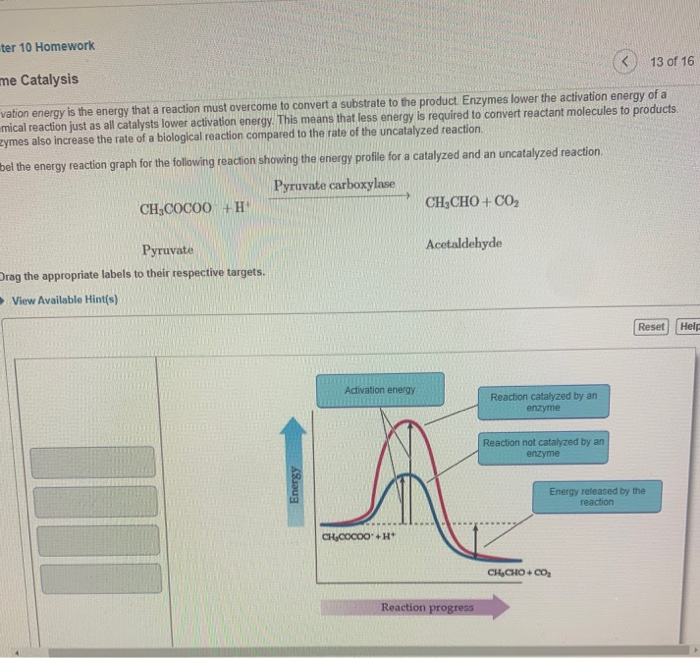
Solved ter 10 Homework < 13 of 16 me Catalysis vation energy









non-tarcisio.png.23bbbfba9c223eb08832cefd988d57dd.png)