
Calcium Carbonate(CaCO3) - Limestone Formula, Structure, Uses
A Computer Science portal for geeks. It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions.
A computer science portal for geeks. It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions.
Calcium Carbonate is a prevalent substance discovered in rocks and shells. Commonly Known as Limestone Calcium Carbonate exists as a white powdery compound composed of calcium carbon and oxygen. The article provides information on calcium carbonate its properties uses commercial production formula preparation types benefits and applications. Table of Content

composition of the CaO catalyst and limestone.
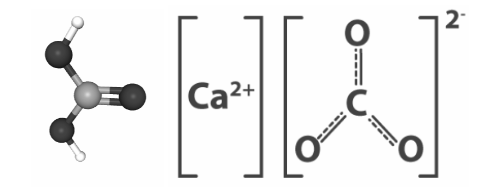
Calcium carbonate formula
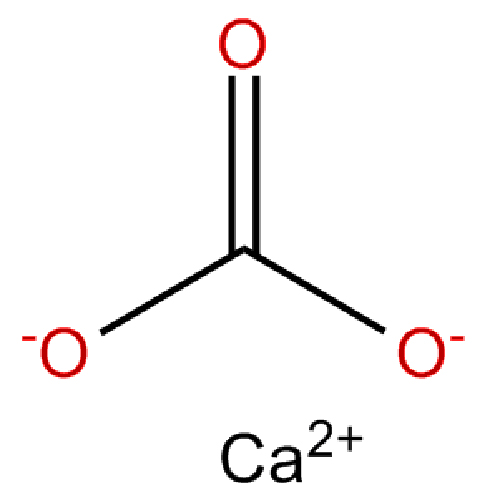
Calcium carbonate - CaCO3
Calcium carbonate

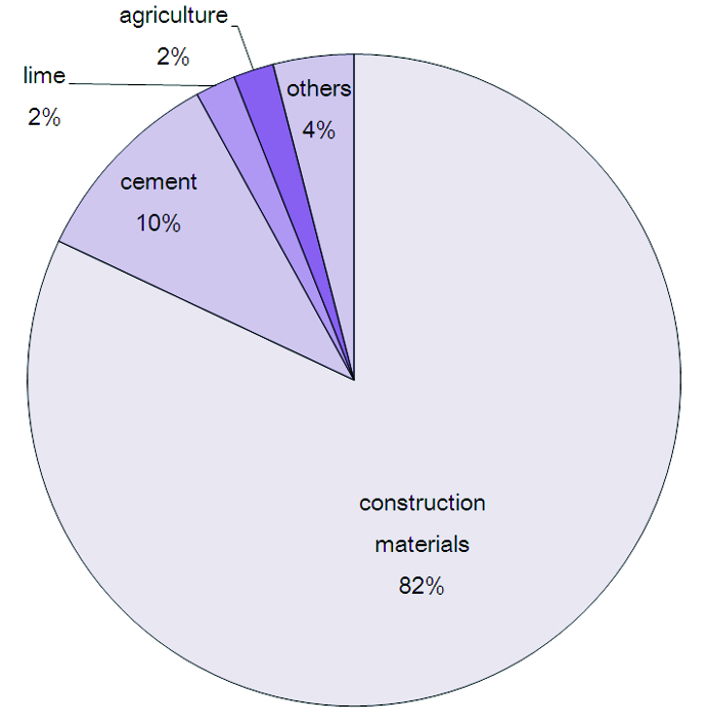
What's the applications for Calcium carbonate(CaCo3)?
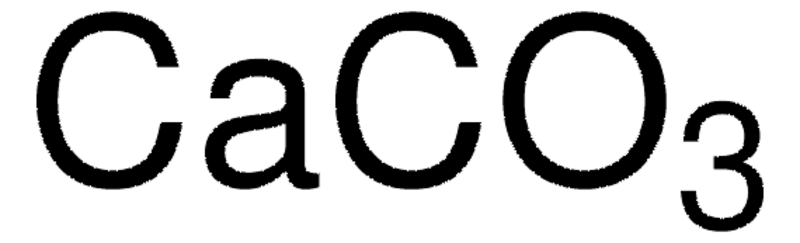
Calcium carbonate ACS reagent, = 99.0 , powder 471-34-1

Schematic of formation of quick lime, hydrate lime, and calcium carbonate

Calcium Carbonate: Definition, Formula, Preparation, Structure, Properties
✓ Solved: Calcium oxide, CaO, is prepared by heating calcium carbonate (from limestone and seashells).
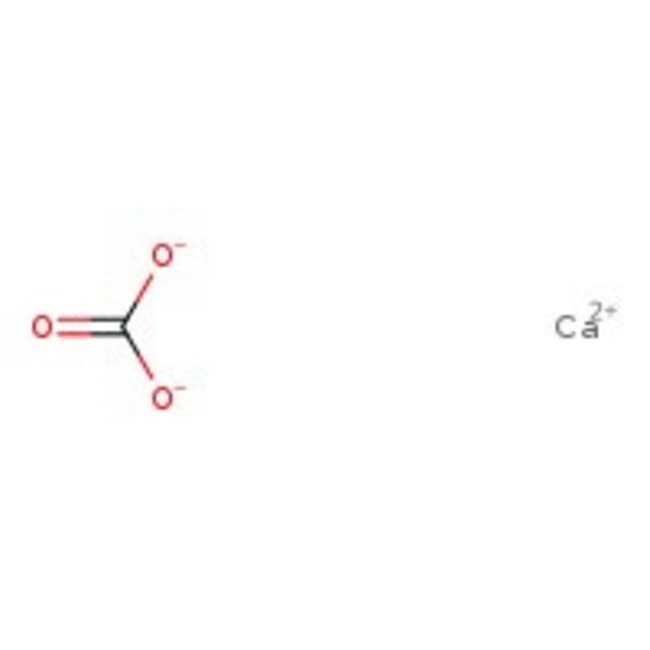
CAS: 471-34-1 - Calcium carbonate

Metastable structures of CaCO3 and their role in transformation of calcite to aragonite and postaragonite
Which elements help in the formation of the formula CaCo3? - Quora
The Chemical Reactivity Of Calcium Carbonate With Oxygen








