Catalysts & Activation Energy
What is a catalyst? Learn all about catalysts of chemical reactions, what is activation energy, and different types of common catalysts.

Catalysts and Activation Energy, 382 plays
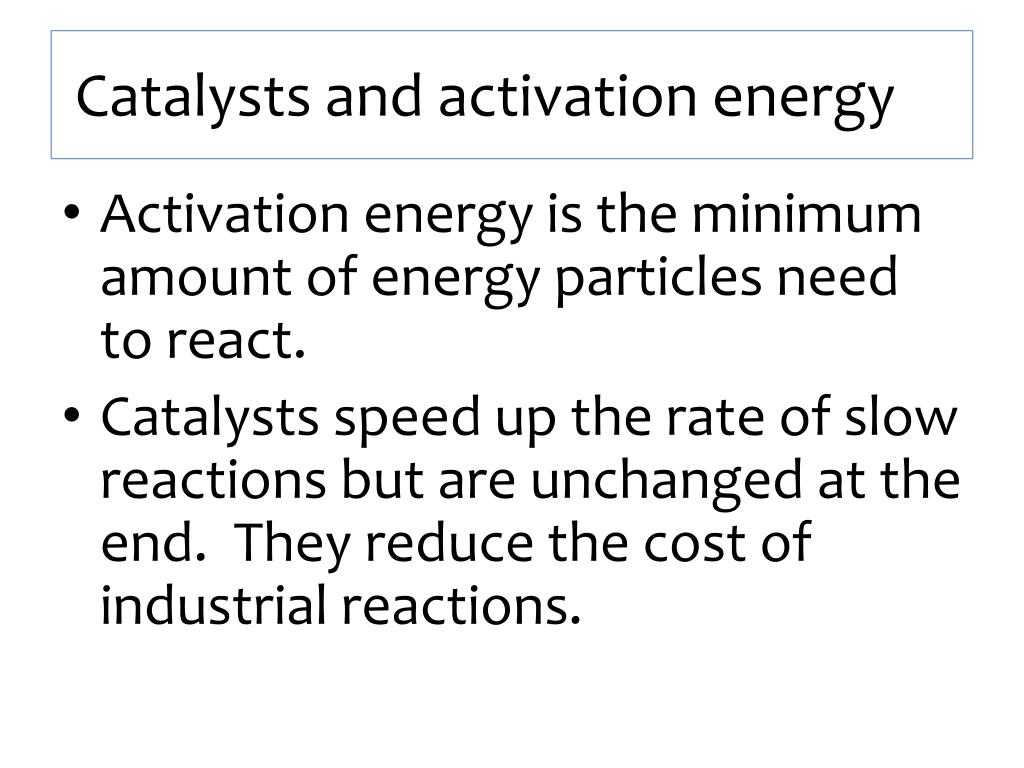
PPT - Chemistry C2 Part three PowerPoint Presentation, free download - ID:2509411

Activation Energy: Definition, Formula, and Graph
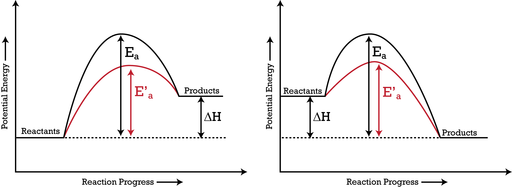
Catalysts Chemistry for Non-Majors

A catalyst decreases the activation energy of a particular exothermic reaction by 39 kJ / mol, to 29 kJ / mol. Assuming that the mechanism has only one step, and that the

What is the “rate of a reaction? The rate of a reaction is the speed of the reaction. It is not “how much” of a product is made, but instead “how quickly”

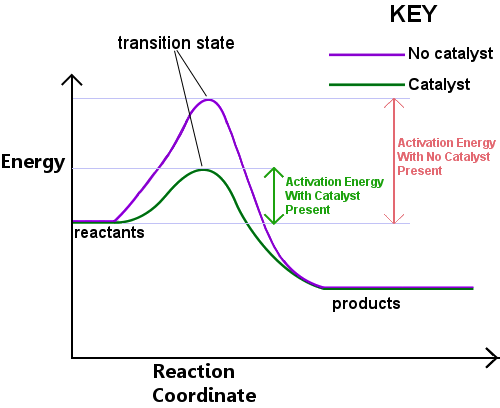
What can a catalyst do and what it cannot do?
Definition of catalyst - Chemistry Dictionary
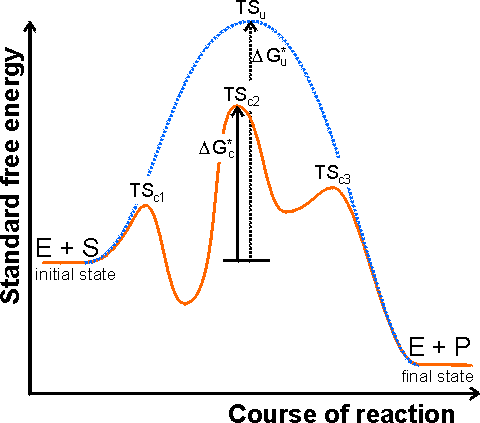
The mechanism of enzyme catalysis

Activation Energy and Catalysts
7.4 Mechanism of reaction and catalysis

Activation Energy and Catalysts, Definition, Relation & Examples - Video & Lesson Transcript

Section 14.5 Activation Energy and Temperature Bill Vining SUNY Oneonta. - ppt download

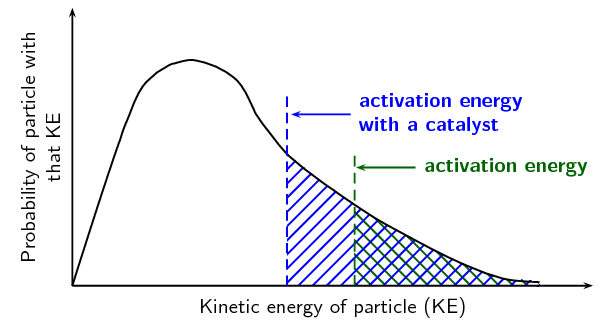
Maxwell-Boltzmann; Temperature and Catalysts - ppt download
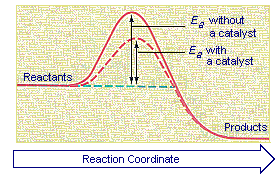
The Activation Energy of Chemical Reactions