Citrate Buffer (0.5 M, pH 3.0)
World Leader in Immunotherapy

pH 4.25 Sodium Citrate Buffer Solution・199-07185[Detail Information], [Analytical Chemistry]
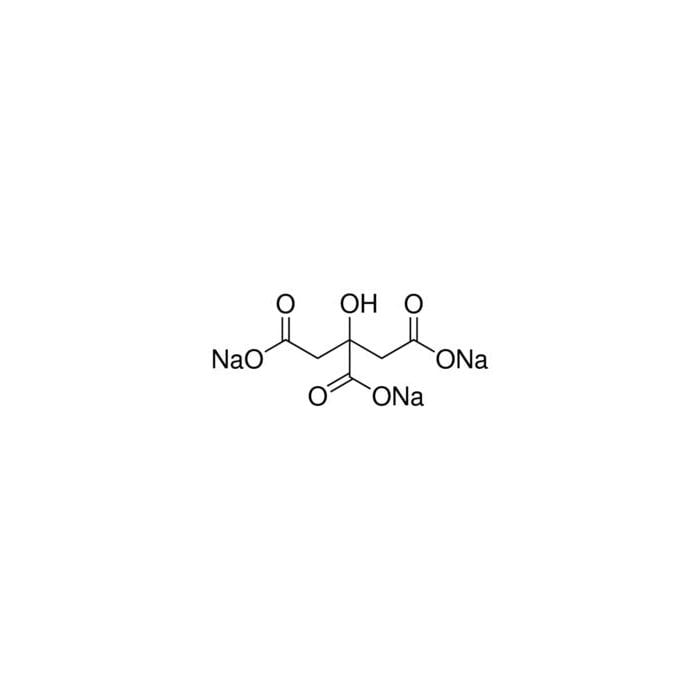
Buffer solution pH 5.00 (20 °C), 33544
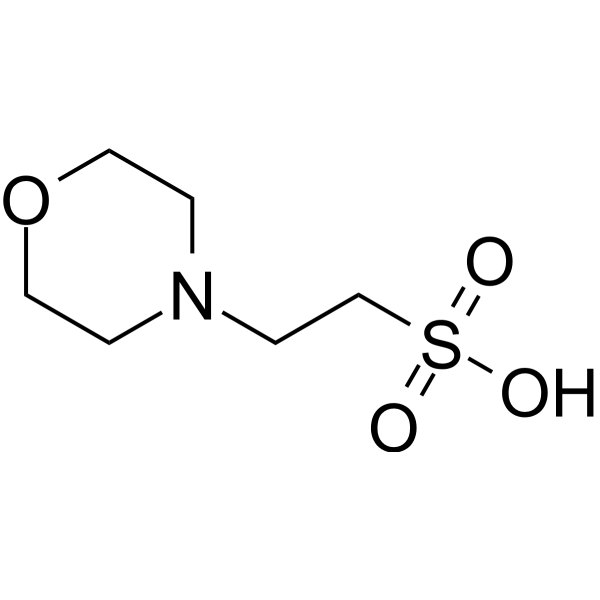
MES (2-Morpholinoethanesulphonic acid), Buffer

PDF) Plastein reaction augments the metal chelating capabilities

Citrate Buffer, pH 6.0, 10×, Antigen Retriever for immunohistochemistry

Citrate Buffer 0.5M, pH 8.0, Sterile

Antioxidant activities and functional properties of grass carp
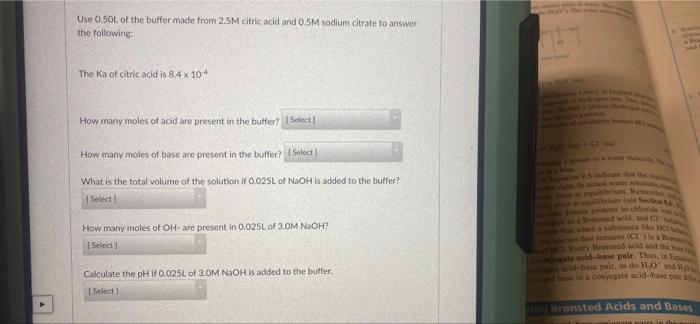
Solved Use 0.5OL of the buffer made from 2.5M citric acid
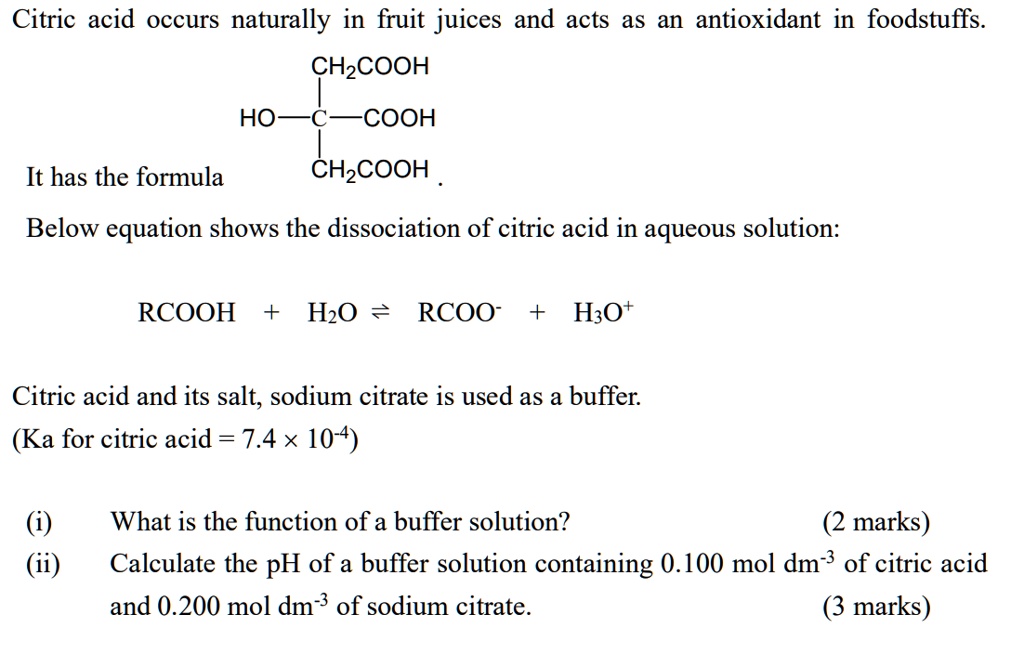
SOLVED: Citric acid occurs naturally in fruit juices and acts as an antioxidant in foodstuffs. CH3COOH HO- COOH It has the formula CH3COOH The equation below shows the dissociation of citric acid

Citrate Buffer, pH 6.0, 10×, Antigen Retriever for immunohistochemistry

Citrate Buffer Ph 6 at Thomas Scientific
How to make a buffer (pH 3) from citric acid, NaOH, and NaCl - Quora

Carbon sources and pathways for citrate secreted by human prostate cancer cells determined by NMR tracing and metabolic modeling

Performing a Separation with Cytiva Products Based on Protein A

Plasmonic Nanosensor Array for Multiplexed DNA-based Pathogen