
FDA Authorizes Modified Risk Tobacco Products
FDA authorized eight snus smokeless tobacco products to advertise with specific information about the lower health risks of using the snus products compared to smoking cigarettes.

In a First, FDA Authorizes Marketing of Low-Nicotine Cigarette as Modified Risk Tobacco Product

FDA authorizes Swedish Match to advertise snus as less harmful than cigarettes

Modified Risk Tobacco Products

FDA Authorizes a Lower Nicotine Cig Advertized to Help Smokers Quit, Bridget Mulroy
Tobacco Education Resource Library Site Search

FDA Authorizes IQOS as a Modified Risk Tobacco Product

U.S. FDA Modified Risk Tobacco Product (MRTP) Application
:sharpen(level=0):output(format=jpeg)/up/dt/2019/11/FDA-grants-first-ever-modified-risk-orders-to-smokeless-tobacco-products.jpg)
DT News - International - FDA grants first modified risk orders to smokeless tobacco products
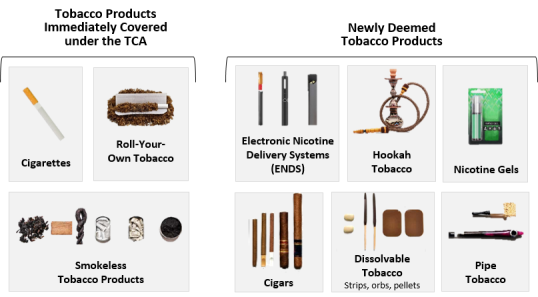
FDA Regulation of Tobacco Products

FDA Authorizes Modified Risk Tobacco Products

Assessment of the exposure to selected smoke constituents in adult smokers using in-market heated tobacco products: a randomized, controlled study

Cuáles son los productos con nicotina de riesgo reducido

These FDA-authorized cigarettes may help adult smokers

Highlights and Insights from the General Snus Modified Risk
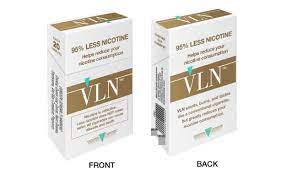
US FDA Gives MRTP to Low Nicotine Combustible Cigarette – Vapor Voice








