Bracco Diagnostics' MultiHance Contrast Agent Earns Expanded Labeling for Pediatric MRI
Bracco Diagnostics Inc. announced the labeling of its contrast agent MultiHance has obtained U.S. Food and Drug Administration (FDA) approval for an extension to include magnetic resonance imaging (MRI) of the central nervous system (CNS) in pediatric patients younger than 2 years of age (including term neonates). The agent may now be used in this patient population to visualize lesions with abnormal blood-brain barrier or abnormal vascularity of the brain, spine and associated tissues.

Perfusion MRI: The Five Most Frequently Asked Technical Questions
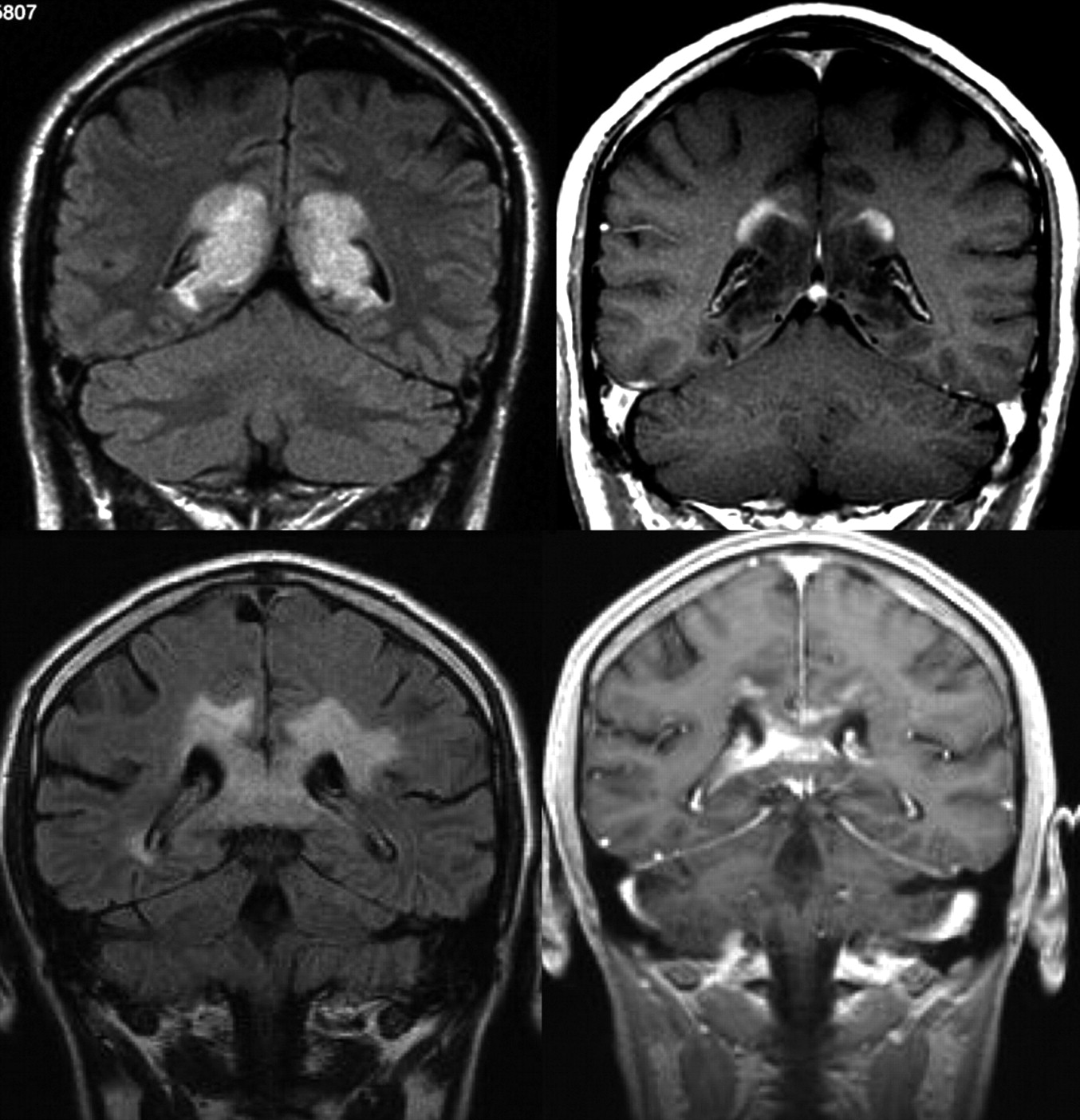
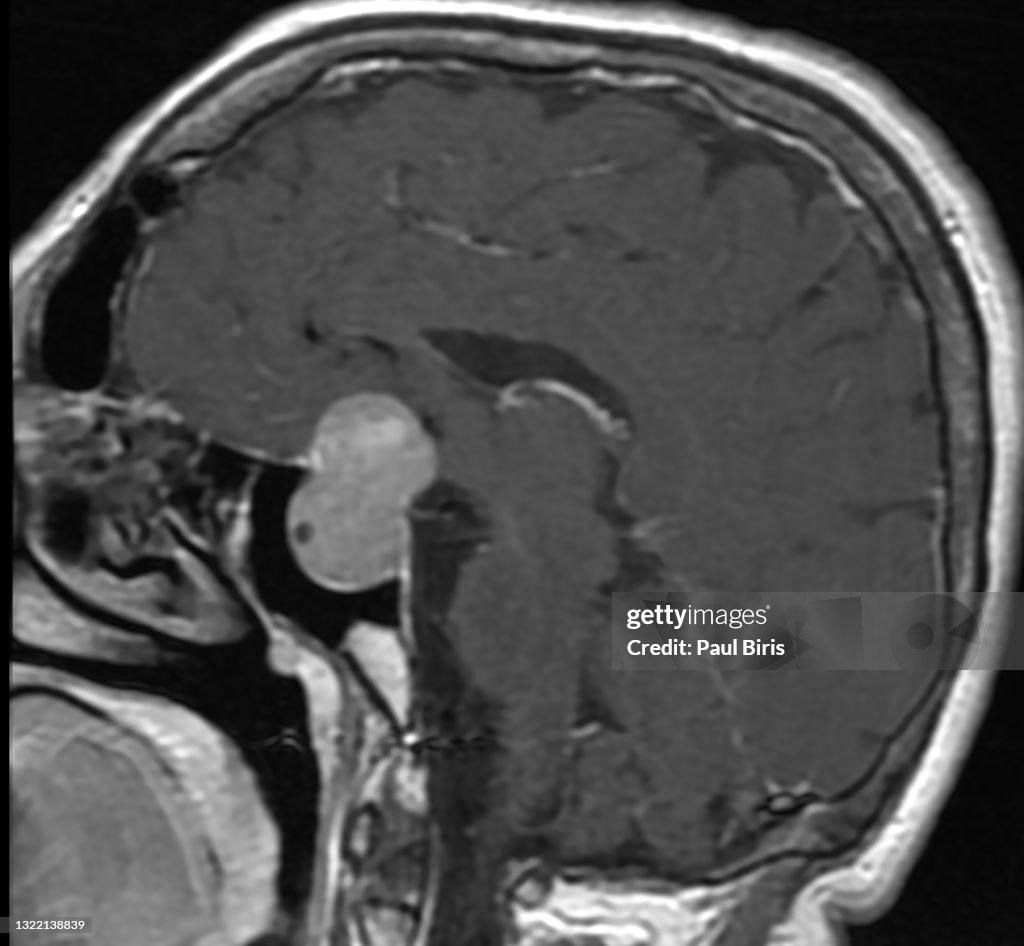
MR Imaging of Neoplastic Central Nervous System Lesions: Review and Recommendations for Current Practice
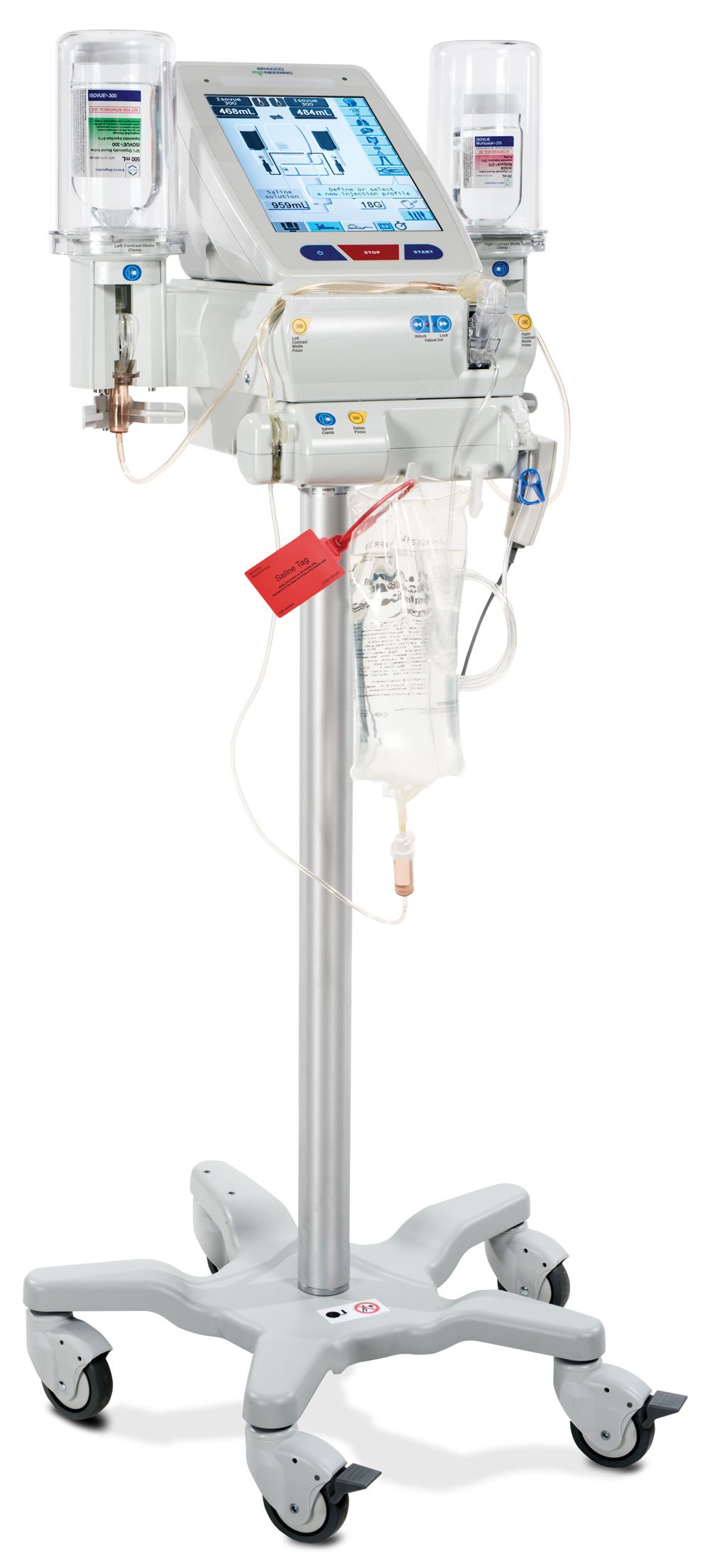
Bracco Launches CT Exprès 3-D Contrast Media Delivery System at RSNA 2016

Gadolinium Retention: A Research Roadmap from the 2018 NIH/ACR/RSNA Workshop on Gadolinium Chelates
Bracco Diagnostics Imaging Technology News

Amidst Increase in MRI Procedures, New Survey Finds 55% of Radiologists Have Concerns About Contrast Agent Availability

Magnetic resonance lymphangiography: with or without contrast? - Abstract - Europe PMC

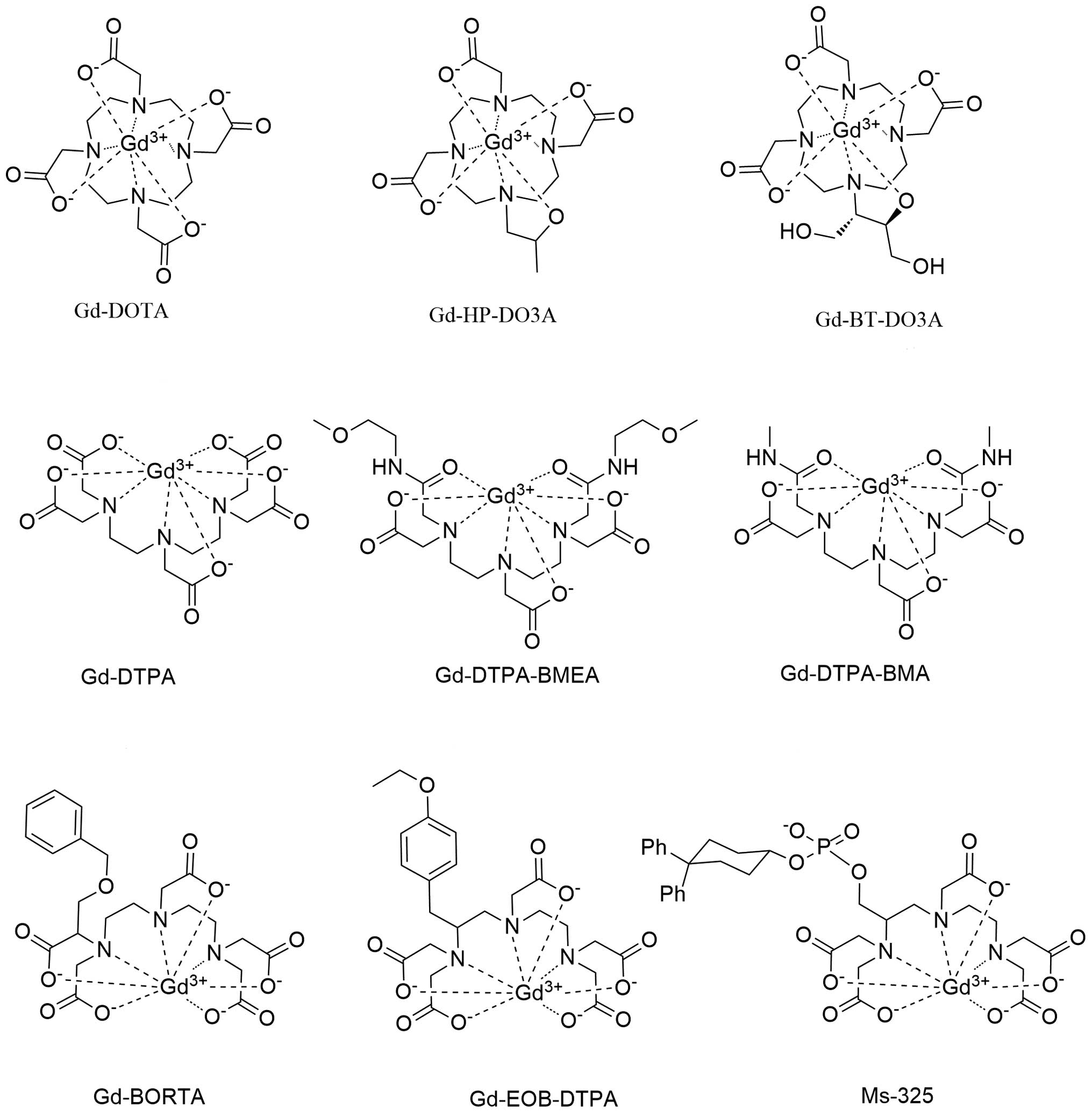
PDF) The utility of gadoteric acid in contrast-enhanced MRI: A review

X-ray-computed tomography contrast agents. - Abstract - Europe PMC

Contrast-enhanced Magnetic Resonance Imaging in Pediatric Patients: Review and Recommendations for Current Practice - Ravi Bhargava, Gabriele Hahn, Wolfgang Hirsch, Myung-Joon Kim, Hans-Joachim Mentzel, Øystein E. Olsen, Eira Stokland, Fabio Triulzi, Elida