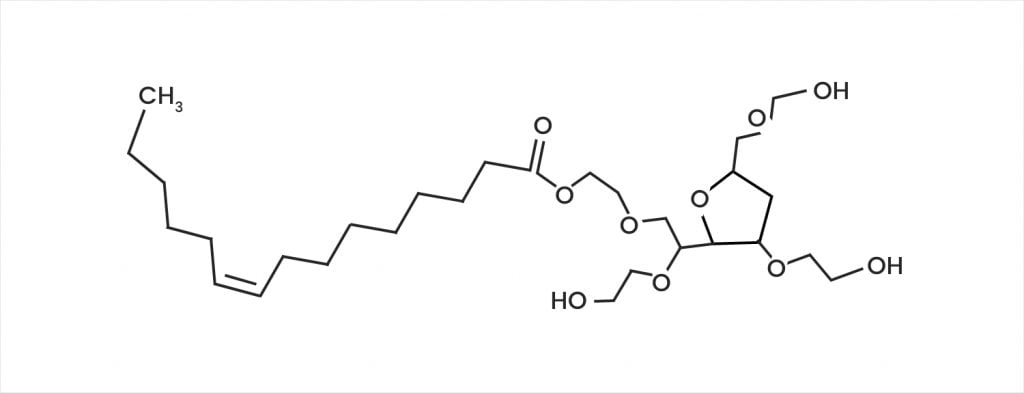
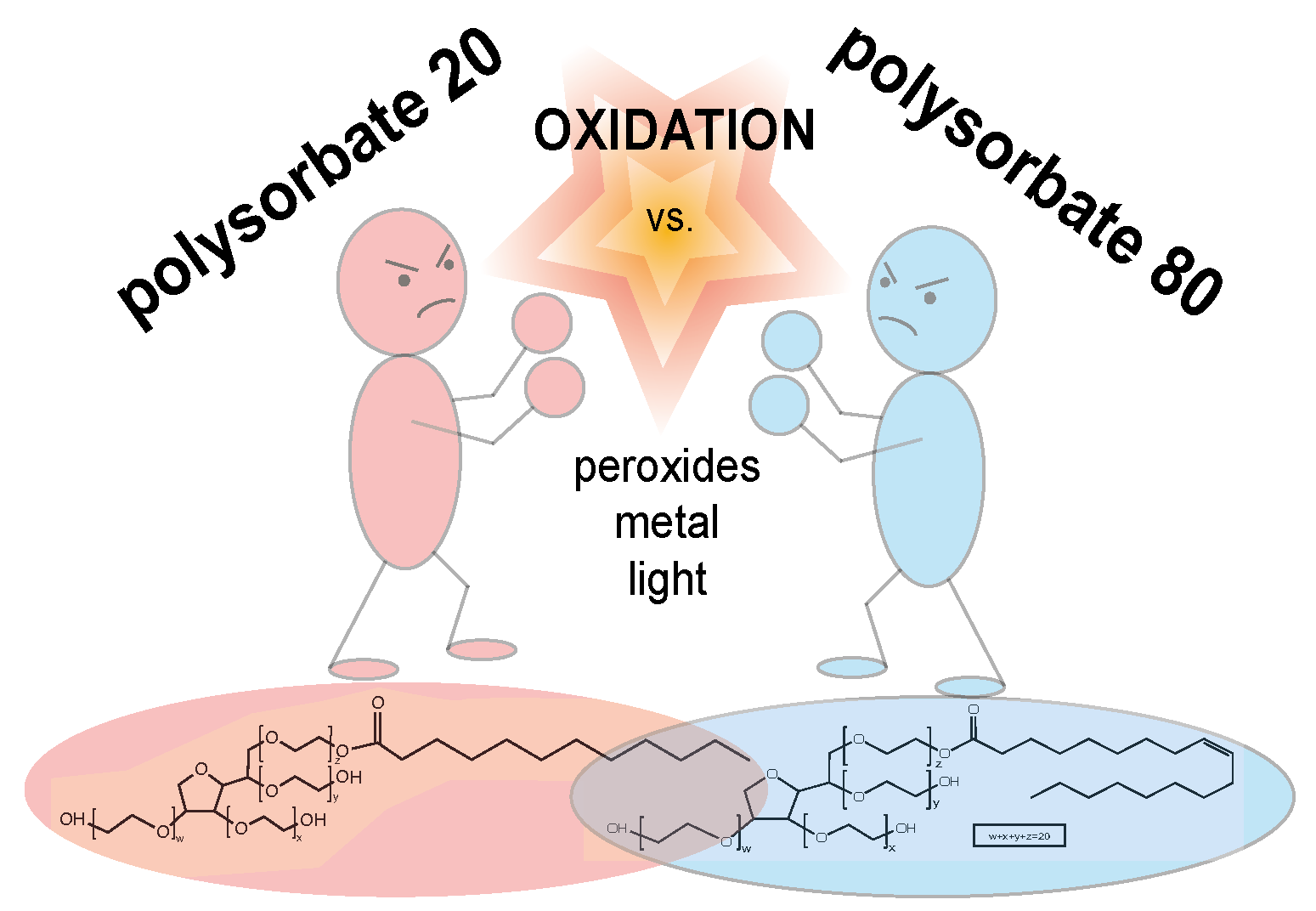
Polysorbate 20 Degradation in Biopharmaceutical Formulations: Quantification of Free Fatty Acids, Characterization of Particulates, and Insights into the Degradation Mechanism

Novel markers to track oxidative polysorbate degradation in pharmaceutical formulations

Profiling Active Enzymes for Polysorbate Degradation in Biotherapeutics by Activity-Based Protein Profiling

Novel markers to track oxidative polysorbate degradation in pharmaceutical formulations

Considerations for the Use of Polysorbates in Biopharmaceuticals

Micellar Morphology of Polysorbate 20 and 80 and Their Ester Fractions in Solution via Small-Angle Neutron Scattering - Journal of Pharmaceutical Sciences

PDF) Quantification of polysorbate 80 in biopharmaceutical formulations implementing an optimized colorimetric approach

Polysorbate 20 Degradation in Biopharmaceutical Formulations: Quantification of Free Fatty Acids, Characterization of Particulates, and Insights into the Degradation Mechanism
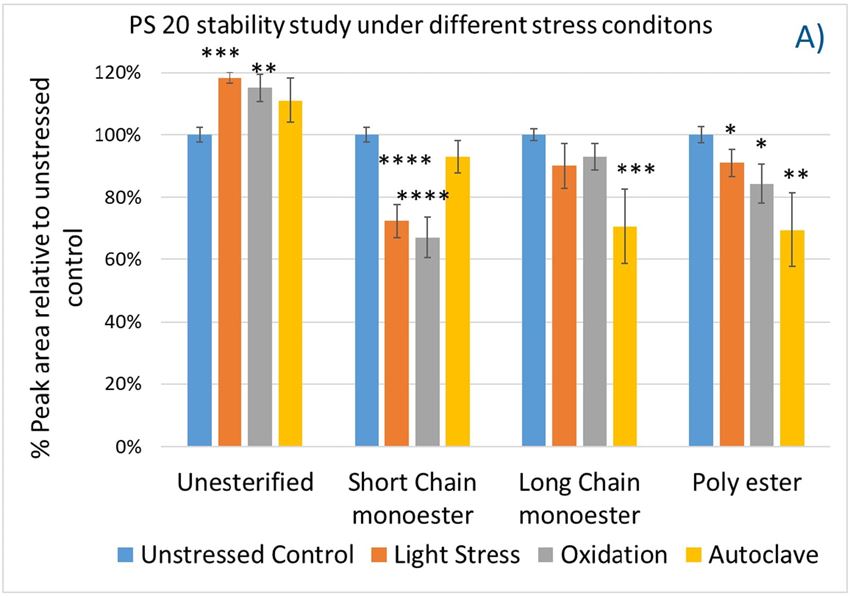
Polysorbates versus Hydroxypropyl Beta-Cyclodextrin: Comparative Study on Excipient Stability and Stabilization Benefits on Monoclonal Antibodies

Identification of the specific causes of polysorbate 20

Understanding Particle Formation: Solubility of Free Fatty Acids as Polysorbate 20 Degradation Byproducts in Therapeutic Monoclonal Antibody Formulations.

Formulation mitigations for particle formation induced by enzymatic
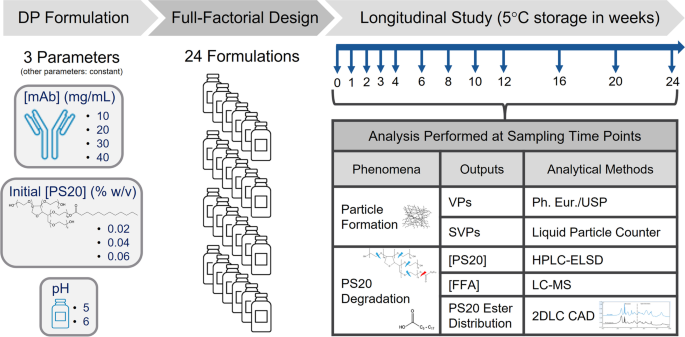
Formulation mitigations for particle formation induced by

Profiling Active Enzymes for Polysorbate Degradation in

Polysorbate degradation in biotherapeutic formulations: Identification and discussion of current root causes