Polymorph screening in pharmaceutical development - European Pharmaceutical Review
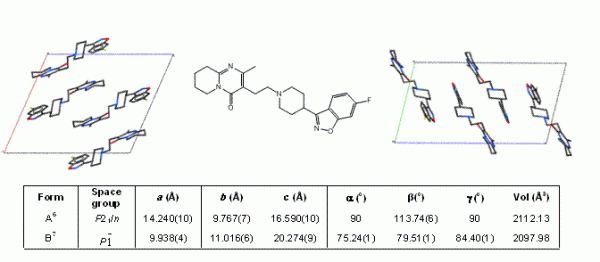
The majority of active pharmaceutical ingredients (APIs) are produced by crystallisation and so the phenomenon of polymorphism, whereby an organic molecule can adopt more than one crystalline form (Figure 1), is of considerable importance when trying to achieve consistent product quality during the manufacture of pharmaceutical solids and solid dosage forms. Although morphology and particle size-distribution are important solid-state characteristics, the uncontrolled occurrence of multiple physical forms (polymorphs, solvates, salts, co-crystals or amorphous) of an API can have significant effects on the performance of the material during processing, manufacture, storage and administration. For example, the solubility difference between some polymorphs has been shown to be over four times that of the least soluble form1 and can vary by significantly more for amorphous forms2.
Polymorph screening in pharmaceutical development - European

A risk-based approach for cell line development, manufacturing and

Unsupervised Pharmaceutical Polymorph Identification and
What are Polymorphs?

Efficient Polymorph Screening through Crystallization from Bulk
Crystals, Free Full-Text

Polymorphs, Solvatomorphs, Hydrate, and Perhydrate of Dabrafenib

A practical guide to pharmaceutical polymorph screening

WO2021032959A1 - Crystalline forms of pyrimidino diazepine derivative - Google Patents
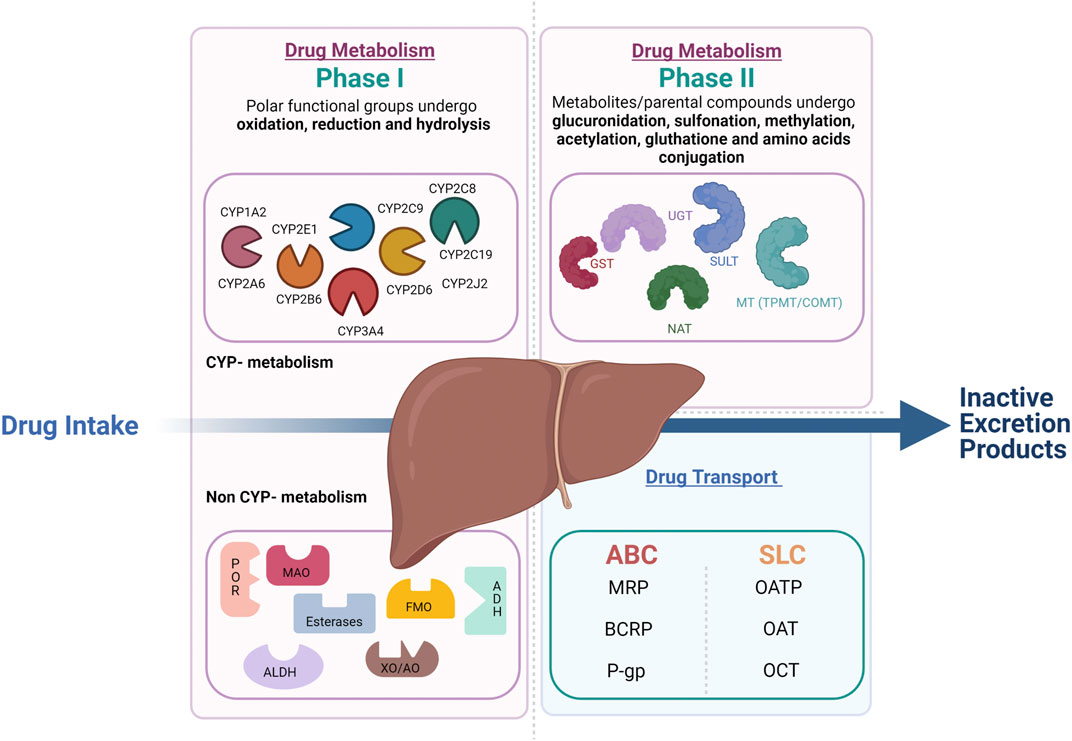
Frontiers Reviewing Data Integrated for PBPK Model Development









