
Scottish NeuroMotor pen gains FDA Breakthrough Device Designation
Scottish tech firm Manus Neurodynamica has been granted Breakthrough Designation by the US FDA for its NeuroMotor Pen device.

Neuromotor Pen – Manus Neurodynamica

Orthopediatric LWW, PDF, Physical Therapy
Neuromotor Pen – Manus Neurodynamica

Andy Stuart on LinkedIn: #hiddendisabilities #snp21 #abuse #hostileenvironment #educate…
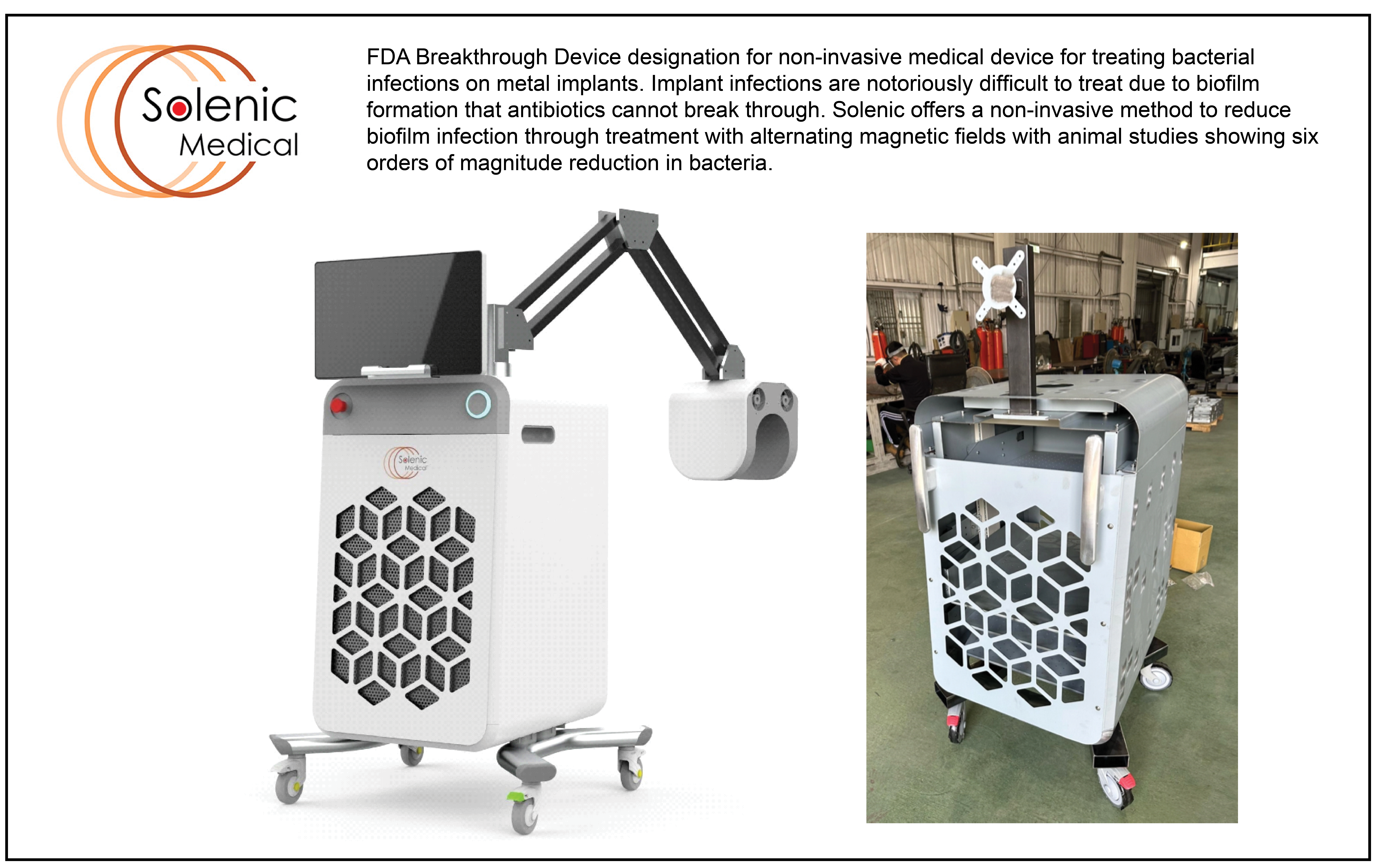
FDA Breakthrough Device Designation for High Impact Medical Devices
nQ Medical's neuroQWERTY Earns FDA Breakthrough Device Designation

Healthcare Weekly Pulse Check Issue #4 Jan 21- Jan 28, 2021

Andy Stuart on LinkedIn: #hiddendisabilities #snp21 #abuse #hostileenvironment #educate…

INBRAIN Neuroelectronics announces FDA Breakthrough Device Designation – Barcelona Institute of Science and Technology – BIST
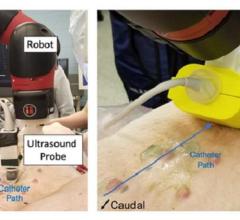
Genetesis Receives 2nd FDA Breakthrough Device Designation for Non-Invasive Diagnosis of Myocardial Ischemia Using CardioFlux MCG
Neuromotor Pen – Manus Neurodynamica

FDA approves Percept PC neurostimulator with BrainSense technology









