Write a chemical equation for the reaction that occurs between stearic acid and triethanolamine under the conditions of the experiment. How does the product of this reaction promote the formation of the
4.7
(187)
Write Review
More
$ 12.99
In stock
Description

Thin-Layer Chromatography

Compare the reaction of 2-chlorobutane with regard to solvent
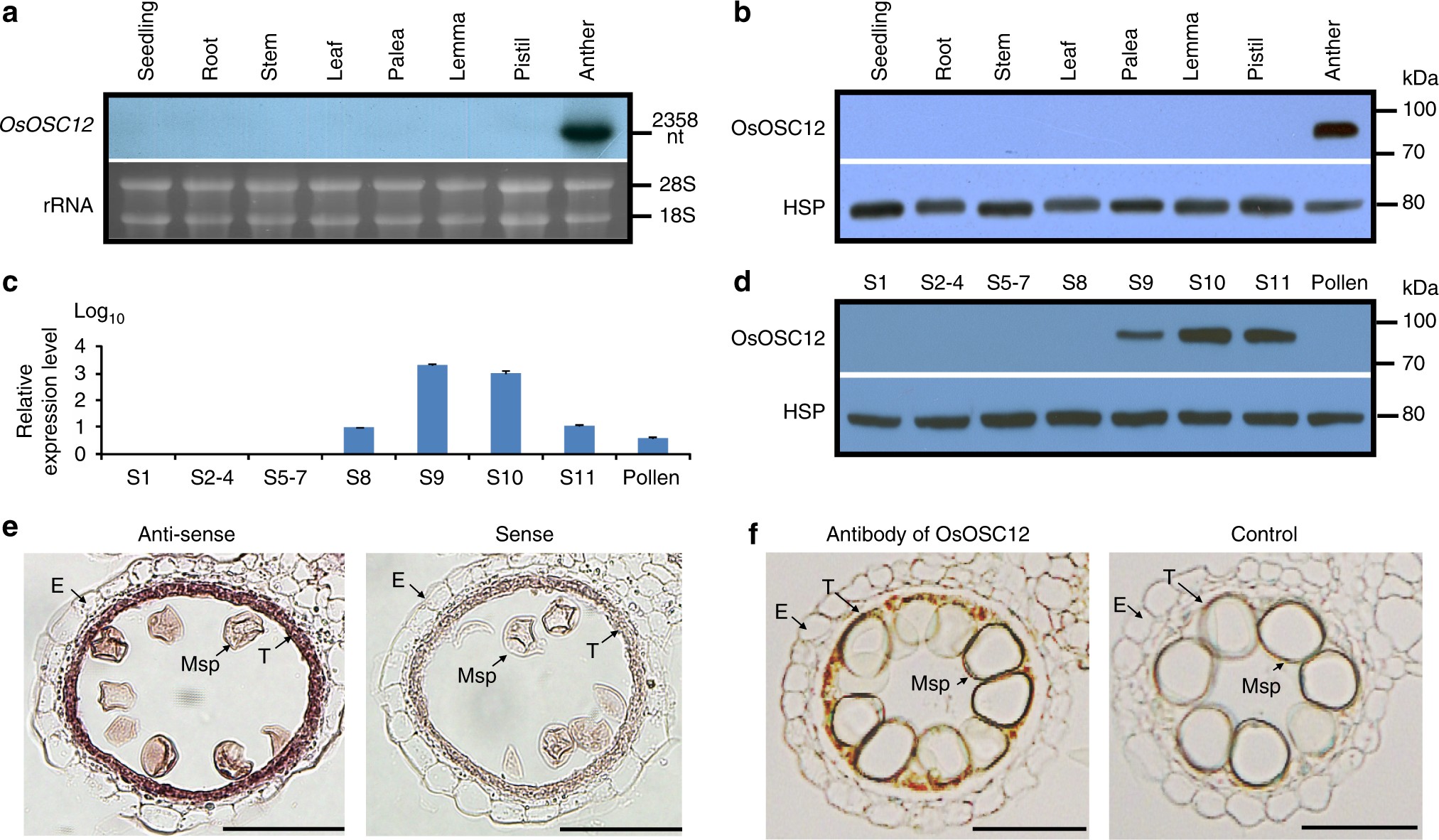
Deficiency of a triterpene pathway results in humidity-sensitive genic male sterility in rice

Principles of Advanced Biochemistry Complete Notes on Lecture Material and Tutorial Notes, BIOC2101 - Principles of Biochemistry (Advanced) - UNSW
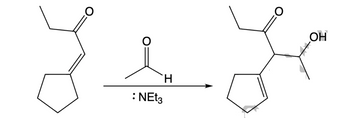
Answered: 1a) Provide the mechanism of the…

Solved DATA Mass of Chips 9.189 Mass of Empty 250 mL Beaker

Identify the mechanisms for the following reaction below as polar

SOLVED: a) Is stearic acid a necessary ingredient for the formation of the hand lotion? What class of organic substance is stearic acid? b) Is triethanolamine a necessary ingredient for the formation

CHEMISTRY
Related products
You may also like