
FDA says Reynolds withdraws modified-risk applications for 6 Camel Snus styles
The Food and Drug Administration disclosed Thursday that R.J. Reynolds Tobacco Co. has requested the withdrawal of modified-risk tobacco product applications for six Camel Snus flavored products.
In Tunisia, more than 50 people have been in prison without trial for months for alleged conspiracy against state security or under a decree punishing the spreading of false information. Most of them are political opponents of President Kais Saied and the Ministry of justice has not been commenting on the cases publicly. For the families and lawyers of the prisoners, waiting for trials is becoming more and more trying. Lilia Blaise, Hamdi Tlili and Fadil Aliriza report.

FDA says Reynolds withdraws modified-risk applications for 6 Camel Snus styles

Newer Nicotine and Tobacco Products: British American Tobacco - TobaccoTactics

MRTP claim authorisation and General Snus sales in the USA: evidence from a difference-in-differences model

Reynolds asks FDA to give Camel Snus products 'modified risk' status - ECigIntelligence
PDF) The Appeal of Smokeless Tobacco Products Among Young Canadian Smokers: The Impact of Pictorial Health Warnings and Relative Risk Messages

FDA authorizes Swedish Match to advertise snus as less harmful than cigarettes

RJ Reynolds Tobacco Co. Seeks Modified Risk Classification for Camel Snus

bti-20221231

FDA spreads confusion about nicotine and smoking - The Counterfactual

Tobacco Truth: FDA Awards “Modified Risk” Status to General Brand Snus Products from Swedish Match
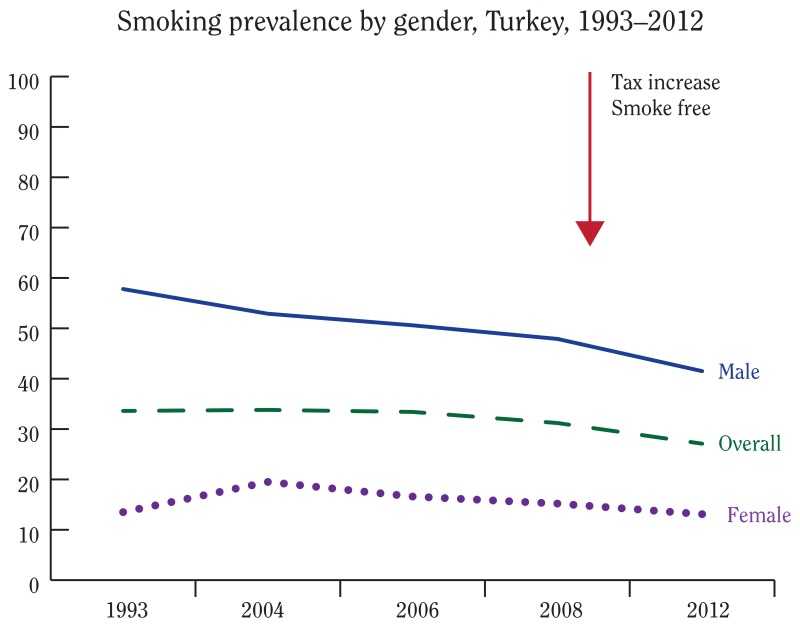
Current Status of Tobacco Control - The Health Consequences of Smoking—50 Years of Progress - NCBI Bookshelf








